What is rust? Rust is a common term used for iron corrosion. When iron comes into contact with water and oxygen (or strong oxidants such as carbon monoxide or strong acids), it rusts. The combination of humidity and temperature with oxygen can also cause rust. It should be noted that oxygen alone cannot cause iron to oxidize, and moisture is also necessary. In general, factors such as humidity, salt water, carbon dioxide and many acidic substances can cause iron rust and lead to the production of iron rust. Rust causes disturbance in the mechanical properties of metal, changes its chemical properties, and in terms of appearance and beauty, the shape of the metal is lost.

Do all metals rust? The question that has occupied many people's minds is: Do all metals rust? All metals are susceptible to rust and corrosion in some way, but the term "rust" is used only for iron, iron alloys and steel. What metals do not rust? Aluminum, brass, bronze, galvanized steel, stainless steel, COR-TEN steel, copper, titanium and special precious metals such as gold, silver and platinum are all metals that do not rust. These types of metals can corrode, stain or tarnish, but they do not rust.

How to prevent rusting of metals? Prevent rust by reducing scratches Metal scratches or cracks hold water. When this happens, water remains in contact with the iron and causes rust. Cold rolled steel is more resistant to corrosion than hot rolled steel. Cold rolling creates a smoother, texture-free surface that traps and holds water. If you notice a deep scratch on the surface of your ferrous metal, you need to fix it as soon as possible. Prevent rust with protective coating applications Immersing metal objects such as watches in a blue colored solution of water, sodium hydroxide and potassium nitrate creates strong corrosion resistance. Commercial rust prevention products, in the form of aerosol sprays or cloth wipes, can also protect metal objects such as tools, outdoor equipment, vehicles and large metal parts from rusting. Prevent rusting with stainless steel Stainless steel alloys contain iron. However, stainless steels resist rust due to their high percentage of reactive chromium content, which is even more reactive than iron. Chromium in the alloy oxidizes quickly and forms a protective layer of chromium oxide on the surface of the metal, which prevents oxygen from reaching the underlying steel; As a result, it will destroy iron rust. Iron rust cleaner with the help of galvanizing process Galvanizing is a coating process used to preserve the underlying steel, providing years of rust-free protection. In the galvanizing process, a piece of steel is coated with liquid zinc on the surface of the iron. Zinc protects steel in three different ways. First, the zinc coating acts as a barrier that will prevent oxygen and water from reaching the steel. Second, even if the coating is scratched, the zinc protects the adjacent areas of the metal through cathodic protection. The third way, zinc is very reactive to oxygen and quickly forms a protective coating of zinc oxide, preventing further oxidation of iron. Prevent rust with regular maintenance Because rust spreads quickly, it's important to scrape it off as soon as it appears. After scraping, wash with warm soapy water and apply a metal conditioner or other protective coating to prevent further oxidation. If necessary, apply a new color layer to the area. Prevent rust by keeping metals dry Rust can be prevented by wiping wet ferrous metals and keeping them as dry as possible. Also, iron objects enclosed in humid environments such as garages or basements benefit from the installation of a dehumidifier. In fact, any kind of mud or dirt that is stuck to the surface can hold water, so keeping metals clean is one of the essential points. Methods of removing iron rust Different methods are used in the industry to remove iron rust. Among others, we can mention sandblasting, shot blasting, water jet, laser cutting, electrolysis, etc. Among other solutions used to remove iron rust, chemical methods such as acid washing and the use of detergents can be mentioned. You can see the most important methods of removing excessive iron rust below. sandblast shot blast water jet lasering acid wash Wire brush sandpaper Citric acid Traditional methods such as white vinegar, baking soda, etc. iron sandblasting Sandblasting is one of the popular and practical methods to remove iron rust, which is a subset of the sandblasting method. In the sandblasting method, surface cleaning is done by natural abrasives and using high air pressure. In the sandblasting method, which is actually a type of sandblasting, sand is thrown at the desired cross-section with high air pressure. In fact, the air acts as a source of power and throws abrasive particles, which are sand and sand, on the surface of the rusty iron to remove the iron oxides on its surface. The sandblasting method is an old method to remove waste materials such as dust, oil and grease from surfaces, which is also used today to remove iron rust. They also use this cleaning method to prepare surfaces for painting. Sandblasting is one of the most popular methods of removing iron rust from different surfaces, which uses natural materials such as silica and metal oxides in different sizes. By controlling the acceleration of the air flow and abrasive materials, different thicknesses of iron rust can be removed from the surfaces. Chemicals are not used in this method and a wide range of waste materials can be eliminated by using it. Despite the countless advantages of the sandblasting method in removing iron rust, in this method the surface may be torn during the operation. Also, breathing abrasive particles in the space during work is harmful to a person's health; Therefore, the sandblasting method also has disadvantages that must be taken into account when choosing. This method is not used for all surfaces, especially soft surfaces.

Shot blast method Shot blasting is another popular method for removing iron rust, which has a nature similar to sandblasting and is included in the category of sandblasting methods. In fact, the only difference between this method and sandblasting is the abrasive materials used. In this method, instead of using sand, silicon metal pellets are used to remove iron rust. The use of metal balls in this method has caused thicker layers of iron oxide to be removed. As a result, compared to the sandblasting method, the spectrum is used more. In addition to using this method to remove iron oxides on parts, it can also be used to improve hardness and create smooth surfaces. Sandblasting can be used for small parts, but this method can only be used for large parts due to the high pressure it puts on the part.

Rust removal with water jet The waterjet process uses high pressure to clean rusted surfaces. In this method, using a high pressure nozzle, the pressure energy of water is converted into kinetic and mechanical energy. This high pressure removes the surface oxide layer from the iron metal surface. Among the components of water jet, we can mention high pressure pump, high pressure hose, high pressure water spray gun, filter and booster pump. When working with water jet, the water pressure is increased up to three times using a high pressure pump. This amount of water pressure, after passing through the filters and reaching the nozzle of the device, is increased up to 2500 times and is ready to be launched. The main advantage of this solution compared to other methods is that it does not cause any surface pollution. It is worth mentioning that the water pressure and flow rate must be fully controlled and checked for the proper functioning of the water jet.


Iron rust laser Using a laser to remove iron rust from surfaces has become one of the most up-to-date methods today, which is able to do this with a small amount of energy. The methods mentioned above are simple methods that are used for surfaces at low and normal temperatures, but in the laser method, tools that work at high temperatures such as jet engines can also be de-rusted. The way of working in this method is that high power rays are irradiated to the desired surface and as a result of this, the metal surface is free from any rust. Among the advantages of iron rust laser, we can mention not using chemicals, high speed, optimal energy consumption and its wide application. Unlike the shot blast method, this method is used for all surfaces, both soft and rough, small and large, and does not damage the surface. The use of laser is not limited to removing rust from irons and this device can also be used to clean the surface of other metals, including copper and aluminum.


Rust removal by acid washing Using acid solutions to remove iron rust and surface cleaning is one of the most widely used methods. Among the acids used in the acid washing method is oxalic acid. In working with this acid, one to three tablespoons of acid are used per gallon of water. Oxalic acid works in such a way that as soon as it is added to metal oxidized with iron oxide, it forms a chemical reaction and forms a colorless ionic compound that is soluble in water. Basically, this acid turns iron rust into an ionic solution and can be easily washed and cleaned with water. The time required for immersing iron parts in oxalic acid solution varies from several hours to several days and depends on the amount of rust and the size of the workpiece. After removing surface oxides using high pressure water flow, iron parts are washed well. In order to ensure that the acid is completely removed from the surface of the piece, the pieces are immersed in sodium bicarbonate solution for several hours. In explaining the reason, it should be said that if acid remains on the surface of the part, it causes corrosion and erosion of the metal. One of the advantages of using the acid washing method is that it can also remove old rust from the surface of the piece. One of the main disadvantages of this method is the excessive consumption of water and the creation of acidic residues at the end of the work, which pollutes the environment.


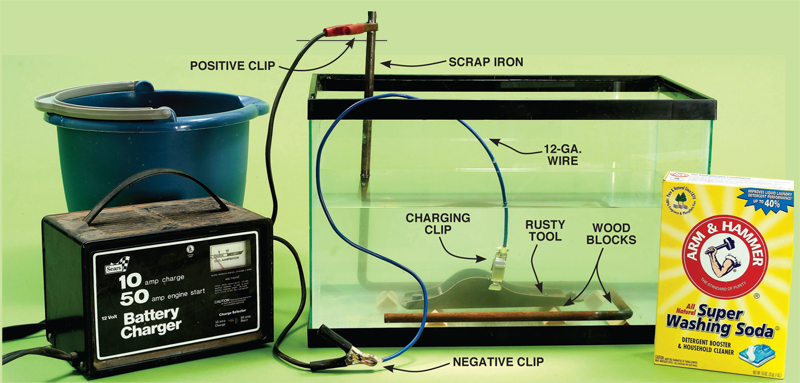
Iron rust removal by electrolysis The method of electrolysis of iron rust can be considered the simplest method of removing iron rust, which can be applied even at home. For example, suppose you need to remove rust from a wrench. In this method, it is necessary to have an electrolyte solution, an anode, cathode and an adapter. For the electrolyte solution, water and baking soda solution can be used, or salt and lemon juice can be used in water. In the next step, a piece of iron should be considered as the cathode and the French wrench as the anode. After that, the electrodes are connected to the cathode and anode and connected to the electricity. After connecting, the iron rusts as an anode are moved to the cathode by the electrolyte and deposited on it. The electrolysis operation may take several days to achieve a good result. Of course, this also depends on the size and amount of rust. After the iron rust is completely removed from the French wrench, wash it well with water to remove the traces of the electrolyte solution from its surface.

Home methods 1- Use white vinegar. The vinegar reacts with the rust to dissolve it from the metal. To use, soak the metal in white vinegar for a few hours and then scrub off the rust paste with an old toothbrush. If the object is too large to soak directly in white vinegar, pour a layer over it and allow time to set. You can also wipe it with a cloth soaked in vinegar. Try soaking aluminum foil in vinegar and using it as a brush to clean the rust. It is less abrasive than a steel brush, but still works to remove rust. You can use regular vinegar and simply let your rusty metal objects soak in it for up to 24 hours before rinsing. This method should not require a lot of washing.
-
2- Try lemon and salt. Sprinkle salt on the rusted area until it is completely covered and apply lime juice on it. Use as much juice as you can and let the mixture sit for 2-3 hours before washing off. Use lime peel to clean the mixture. It is strong enough to remove rust without further damage to the metal.
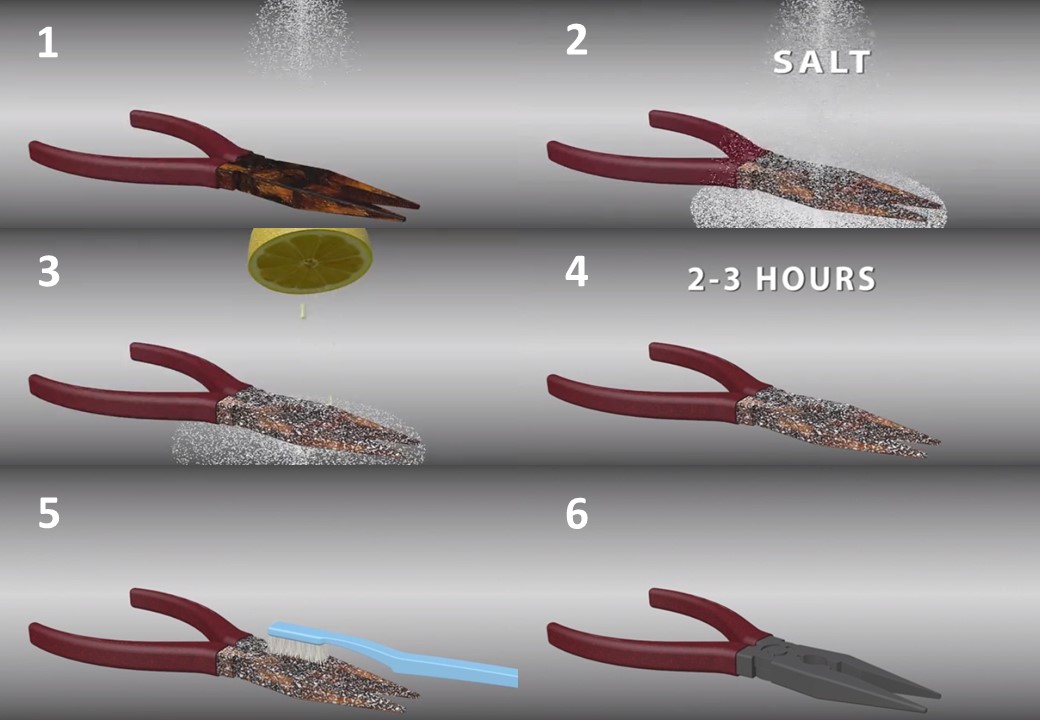
-
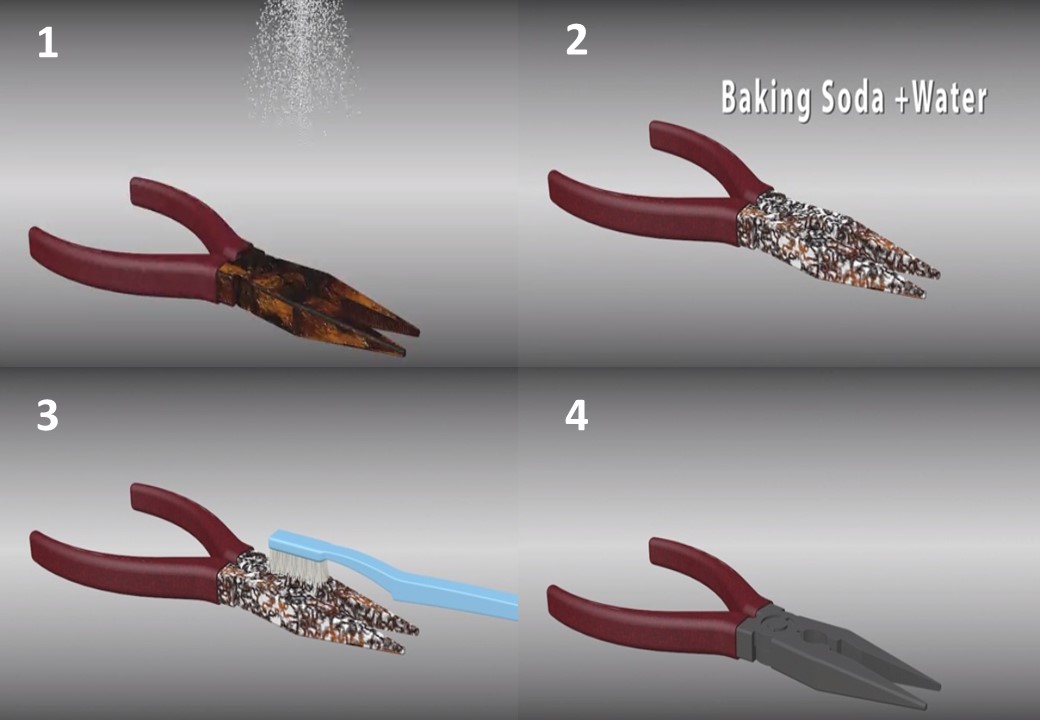
3- Make a paste using baking soda. Mix baking soda with water until thick enough to spread on the metal. Allow time to harden and then clean with a soft brush. Try using a toothbrush to remove the baking soda and rinse with water. The baking soda mixture can be diluted as much as you want, there is no exact recipe.
-
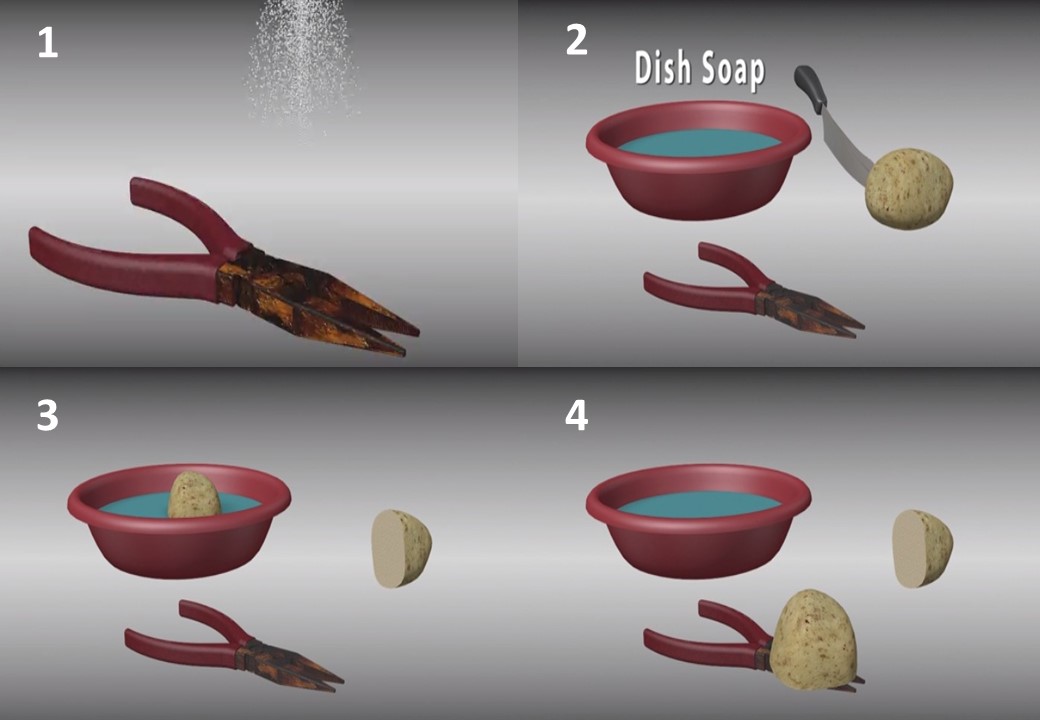
4- Try to use potatoes and dishwashing liquid. Cut the potato in half and cover the cut end with dishwashing liquid. This creates a chemical reaction with the rust, making it easier to remove. Place the potatoes on the metal and let it sit for a few hours. To reuse, simply remove the used end of the potato, add more dishwashing liquid, and let it soak longer in the metal.
-
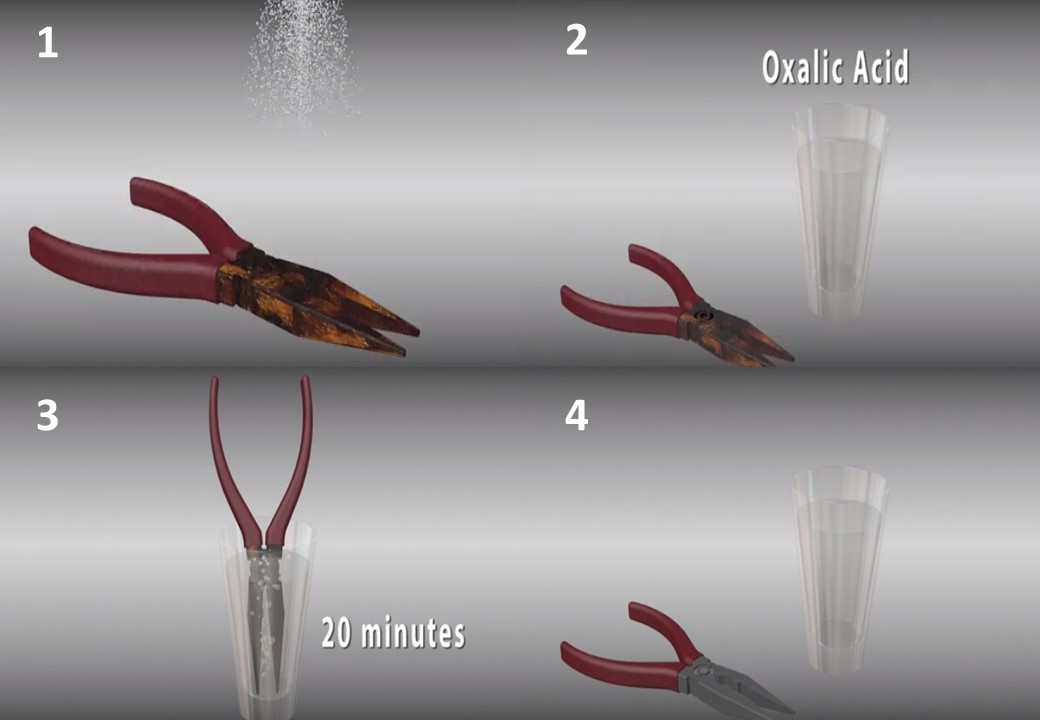
5- Use oxalic acid. Take precautions with this method - use rubber gloves, goggles and protective clothing. Avoid direct inhalation of acid gas. Wash the rusted item with detergent and dry carefully. Mix about 25 ml (one teaspoon of 5 ml) of oxalic acid with 250 ml of warm water. Soak the item for about 20 minutes or clean it with a cloth or brass brush. When the rust is finished, rinse and dry thoroughly.