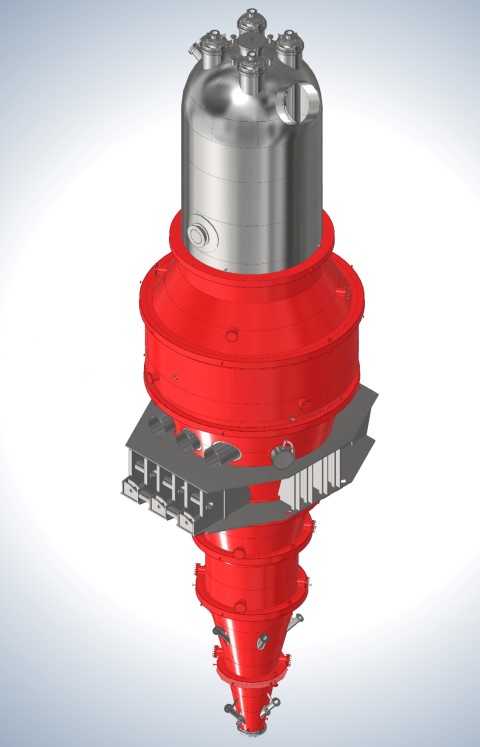
Direct regeneration, production of sponge iron in solid state

The environmental disadvantages and energy consumption of smelting caused the emergence of a new method in iron production called direct reduction. In this method, the agent of reduction and conversion of iron oxide to metallic iron is natural gas and the direct reduction process occurs in the solid state. In this method, iron ore is ground into pellets (Pellets) It enters and enters the reactor.
Natural gas is also after being cracked and converted into H2 و CO It enters the reactor and the pellet (iron oxide) turns into sponge iron (metallic iron) after reduction in solid state. In direct regeneration, the percentage of metallization (the rate of conversion of existing iron oxide to metallic iron) is high ۹۰percent but for use and steelmaking, sponge iron pellets to electric arc furnace (EAF) They are transferred and after removing the impurities and reaching the right composition, they are prepared as primary products such as slabs.
Watch the sponge iron regeneration process in full in the video linked below.
https://drive.google.com/file/d/1jkc1xAcilL2lVvHmT_gby272lsgPzExP/view?usp=drive_link
History and necessity of direct revival
Direct recovery of sponge iron compared to the old and traditional method of iron production by blast furnace has a short history ۵۰is years old Iron production through blast furnace has many advantages that no method has been able to replace it yet ۹۴The percentage of iron in the world is produced by that method, but reasons such as environmental issues and the reduction of coking coal reserves caused new methods of iron recovery to be taken into consideration. The first direct recovery or production of sponge iron by American and Mexican researchers in a decade ۷۰It took place .
Direct reduction reactions are generally below the melting point of iron and ca ۸۲۰degrees Celsius, but in the blast furnace due to the high heat capacity of the coke, the product is in molten form, on the other hand, the dissolution of the carbon in the coke produces the product in the form of crude iron with a high carbon percentage, which requires more energy in the steelmaking stage. The initial investment and operating costs of sponge iron plants are low compared to integrated steel plants and are suitable for developing countries where coking coal resources are limited. .
Direct regeneration mechanism
The regeneration process takes place in direct regeneration methods, such as the one that occurs in the belly of the blast furnace, and the regenerating gas acts on the iron oxide and regenerates it in several stages. In this method, the iron oxide feed is in the form of porous pellets so that it has the ability to pass the reducing gas .
Regenerative gas in direct reduction reactors before entering the reactor, in reformers from the cracking of natural gas (CH4) and become CO و H2 It is prepared and after preheating to temperature ۱۰۰۰degrees centigrade enter the direct reduction reactor. In direct resuscitation methods at least ۹۰%The iron oxide in the pellet has been converted into metallic iron, and the ratio of the metallic iron formed to the total iron in the pellet is called metallization percentage. The product of the direct reduction reactor, which is a solid sponge iron, is readily available at a temperature of approx ۶۰۰degrees Celsius by the material transfer system directly to electric arc furnaces (EAF) It is charged and refining and steel making is done on it.
Microscopic image of sponge iron
In general, there are the following steps in the path of iron ore to steel :
1. Concentration (mining and concentration):
Most of the iron ore is extracted by surface mining. There are some underground mines, but wherever possible, surface mining is preferred because it is cheaper. On average, the grade of ore is around 60 to 65%, concentration and increase of ore grade occurs depending on its initial grade, which includes crushing, grinding, and physical and magnetic methods. The product of these processes, which has a higher percentage of iron than ore, is called concentrate. Crushing and screening are simple mechanical operations that do not change the composition of the rock, but some rocks need to be upgraded before smelting. Most concentrator processes rely on density differences to separate lighter minerals from heavier ones, in some cases to separate fine iron ore from impurities.
Most of the iron ore is extracted by surface mining. There are some underground mines, but wherever possible, surface mining is preferred because it is cheaper. On average, the grade of ore is around 60 to 65%, concentration and increase of ore grade occurs depending on its initial grade, which includes crushing, grinding, and physical and magnetic methods. The product of these processes, which has a higher percentage of iron than ore, is called concentrate. Crushing and screening are simple mechanical operations that do not change the composition of the rock, but some rocks need to be upgraded before smelting. Most concentrator processes rely on density differences to separate lighter minerals from heavier ones, in some cases to separate fine iron ore from impurities.
2. Threading:
The ore or concentrate is in the form of a very fine powder, which is not suitable for use in a reaction furnace, so the concentrate must be agglomerated with pelletizing agents. Wet concentrates are fed into an inclined and rotating disc, which vibrates to produce soft, spherical agglomerates. These green agglomerates after drying and burning at a temperature limit of 1250 to 1340 degrees Celsius. Finally, they cool down slowly. The pellets of the final product are round and have a diameter of 10-15 mm, and they form an almost ideal shape for the furnace.
3. Iron making:
At this stage, inside iron production furnaces based on direct reduction mechanism, oxygen is removed and sponge iron is prepared. At first, iron ore is in the form of hematite (Fe2O3), then its percentage of oxygen decreases to the level of magnetite (Fe3O4) and finally to the level of the percentage of oxygen in westite (FeO), and finally metallic iron is formed.
4. Smelting and steelmaking:
The sponge iron pellets are transferred to the electric arc furnace (EAF) and after removing the impurities and reaching the appropriate composition, they are prepared as primary products such as slabs. Here, scrap iron is first poured into the electric arc furnace using special baskets, and then, at the same time as the scraps are melted, a combination of sponge iron and slag-forming materials such as lime, coke, bentonite and other additives are added to the melt from the top of the furnace. And after sampling, oxygen blowing and homogenization, as well as various analyses, it turns into molten steel. After discharging the slag, the melt mixture is sent to the continuous casting unit by the melt transport bottles. In the continuous casting unit, the melt is poured into containers called tandish through a nozzle and then into a water-circulating copper mold, and along the path of a roller it is sprayed with cool water and turns into a frozen ingot. Finally, the produced slabs are cut to the desired lengths. It is produced from slabs after metallurgical processes of sheets and other steel sections. The first company that worked in the field of sponge iron production was the Midrex Company of America, which is still producing with this method and under the name of this company in most parts of the world, which accounts for 63% of the production of sponge iron. Another group that has been active in the production of sponge iron is the Mexican company HYL. This company now claims that it has been able to market the fourth generation of its furnaces by eliminating reformer gas. Other sponge iron production methods include SL/RN, Jindal, DRC, which are based on coal, and the innovative method in Iran called PERED.
Purofer method
The Purofer method is one of the methods used in the production of sponge iron. In this method, iron ore must first be sieved and the material obtained must be poured into a cone-shaped furnace. In this process, iron ore flows down and natural gas along with an auxiliary gas are moved up.
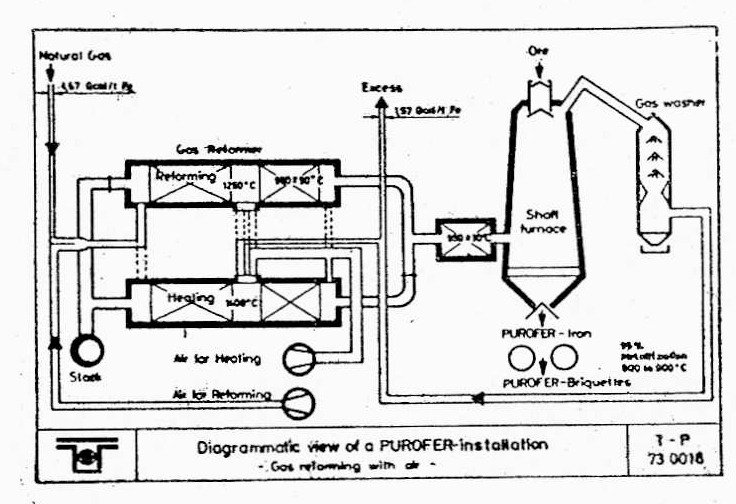
This method has the ability to recover up to 95% of the iron in the pellet. In this method, sponge iron is obtained, which has suitable characteristics and properties for use in the steel industry. In the Purofer method, using its unique process, it is able to regenerate iron effectively and with high efficiency. This method creates a significant improvement in the quality and final product of sponge iron and is suitable for the production of sponge iron with excellent quality and high usability. By providing an optimal method for the production of sponge iron, the Purofer method plays an important role in improving the steelmaking processes and increasing the efficiency of the related industry. By using this advanced method, it is possible to achieve the production of sponge iron with high quality and better efficiency and meet the various needs of the steel industry.
The teacher's method
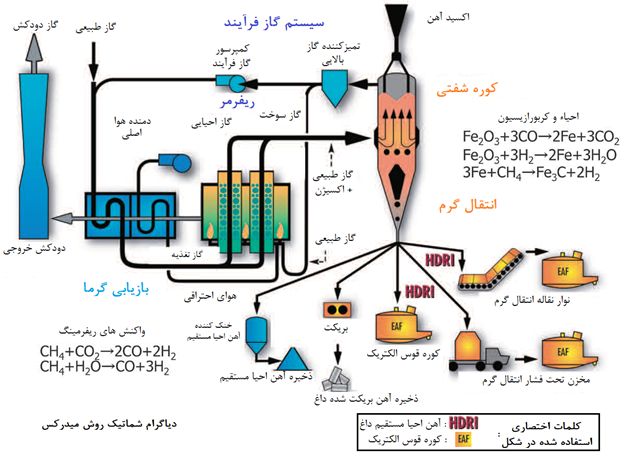
The most widely used and most productive process at present is the direct regeneration of pellet iron ore and the production of sponge iron by gaseous method. In this method, the regenerator is natural gas, which is usually considered to be methane (CH4). (Approximately 85% of natural gas is methane, so natural gas is assumed to be approximately methane) In this process, the pellets fall from the upper part of the furnace to the bottom, and during this period of time, regenerative gas is blown into the furnace. As mentioned, the mixture of hydrogen gas and carbon monoxide is prepared from breaking and decomposition before entering the furnace in the reformer unit. Due to the natural flow of the hot fluid (1000°C), the regenerating gas moves upwards and meanwhile the percentage of oxygen in the falling pellets is reduced and the final product is sponge iron. In general, the route of direct recovery for an iron ore is as shown below. In addition, a part of the gas has always ascended into the furnace and will be used again as fuel (because of the amount of carbon oxide and hydrogen), while some of this gas is returned, it can participate in the methane oxidation process. have The Midrex method has many advantages, such as the fact that this method has been so widely used among sponge iron producers (by gas method) (about 60%) that it is now considered as an accessible technology. On the other hand, the reduction of repair costs in this method due to the greater simplicity of the system and the high efficiency of the production sponge (94-96% metallization) has made it a suitable platform for investment. Another point is that the sponges produced by the Midrex method have more uniformity in terms of chemical composition. Of course, the Medrex method, with all its advantages and merits, also has its weaknesses. The sponge iron produced by the Midrex method is harder than other methods, and this means more initial energy is required during steelmaking. Another important point is the erosion of the body of the regeneration cord over time, which, of course, has been partially resolved in recent years with proper insulation of the furnace.
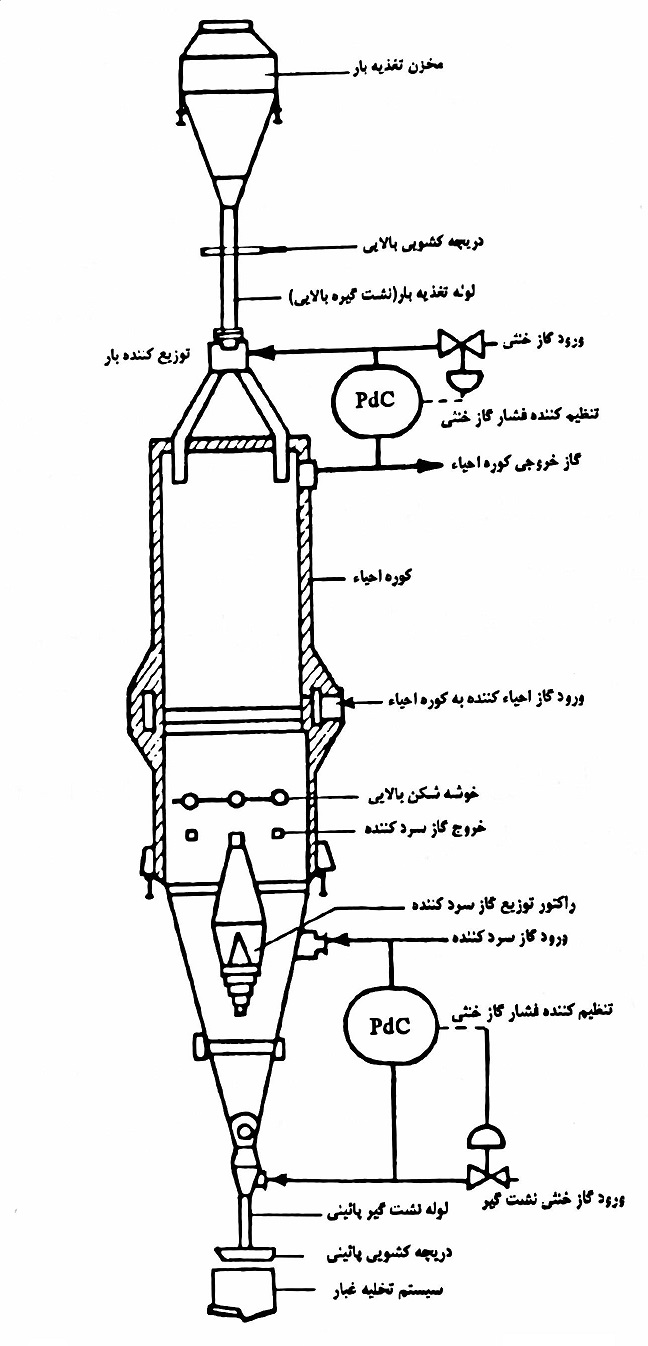
method HYL
This process was designed as an experiment in the beginning of the 50s with a nominal capacity of 50 tons per day. The first problem during the tests was not achieving the required amount of regeneration during the process, after 18 months of research and investigation, finally after applying several changes, one of the most important of which was the change in the regenerative gas system, the first iron ore regeneration system and production Sponge iron by HYL method was launched as an experiment in the middle of 1955. The experiments were successful and the production capacity of sponge iron was gradually increasing so that in the last days of the research of this process, the system was able to produce 60 tons of sponge per day in an optimized way. After the success of the experimental system, the designers sought to expand this model and commercialize it, until in the last days of 1957, the first commercial plan for the production of sponge iron by the method of direct recovery of iron ore started working, a plan known as HYLSA Monterry 1-M. It had a capacity of 75,000 tons per year. The process was successful and the Monterey project continued its operation for 35 years. In 1978, Monterey's design also received historical approval when ASTM considered this design as the first successful industrial design in the production of sponge iron. Despite the success of the HYL process in the production of sponge iron, it still has its own weaknesses. One of these weaknesses was the static nature of the production process platform, which greatly reduced the ability of this process to compete with other processes. For this reason, in the following years, the HYL III plan was established, the basis of which was to change the production process from a static bed to a production process with a moving bed.
HYL process equipment
The intended furnace for recovery operations, which has a charging and discharging system for the produced sponge iron. The regeneration gas system, which itself includes many parts, including the heating unit, cooling, adjusting the required gas pressure, humidity, etc. Conveyors needed for transporting and moving primary iron ore as well as final product transportation system (production sponge) water cooling system used in the process; This system is a closed thermodynamic cycle. Process control unit Process electrical feeding system Cooling tower with desired filtration CO2 removal unit
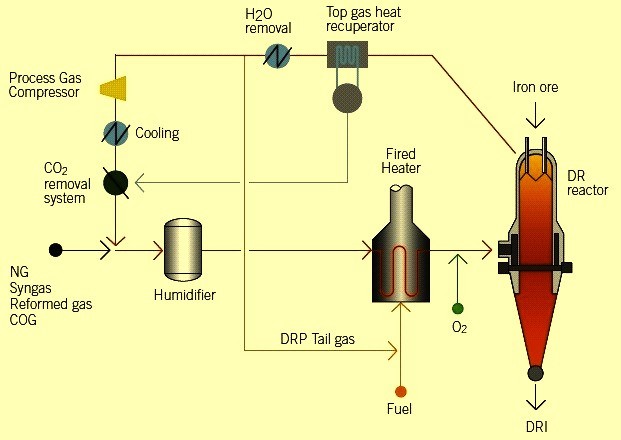
The figure above is a simplified schematic of the HYL process. In this process, similar to the Midrex system, it uses H2 and CO as a regenerative gas to produce DRI. The range of product production in this process is three categories of DRI, cold DRIs and HBI.
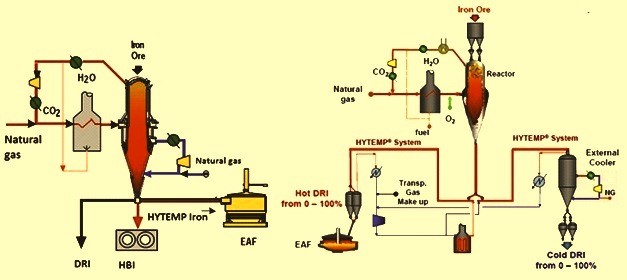
The above schematic figure shows how to produce the three products that can be produced in the HYL process. Cold DRI is usually produced for use in steel production factories near the sponge iron production site. Meanwhile, HBI production is mostly used for export purposes. Cold DRI is usually produced under the processes Oxidation is prevented, while it can also be exported. HYTEMP iron is also commonly used for direct use in electric arc furnaces in steel factories.
Production mechanism
-
This process consists of two general parts, which include the regeneration gas production and preparation unit and the regeneration furnace unit. The required gas is obtained from the reactions between natural gas (methane) and existing water vapor. The important point is the carburization of the sponge iron produced during this mechanism, which is caused by the chemical reaction with the same gases in the system. The primary reactions for the production of reducing gases are as follows:
2CH4+ O2→ 2CO + 4H2- CH4+ H2O → CO + 3H2
- 2H2+ O2→ 2H2O
- CO2+ H2→ CO + H2O
- Fe2O3+ 3CO → 2Fe + 3CO2
- Fe2O3 + 3H2→ 2Fe + 3H2O
-
3Fe + 2CO → Fe3C + CO2
-
&
- 3Fe + CO + H2 → Fe3C + H2O
-
Why did we talk about theory?! The reason is in the following reactions:
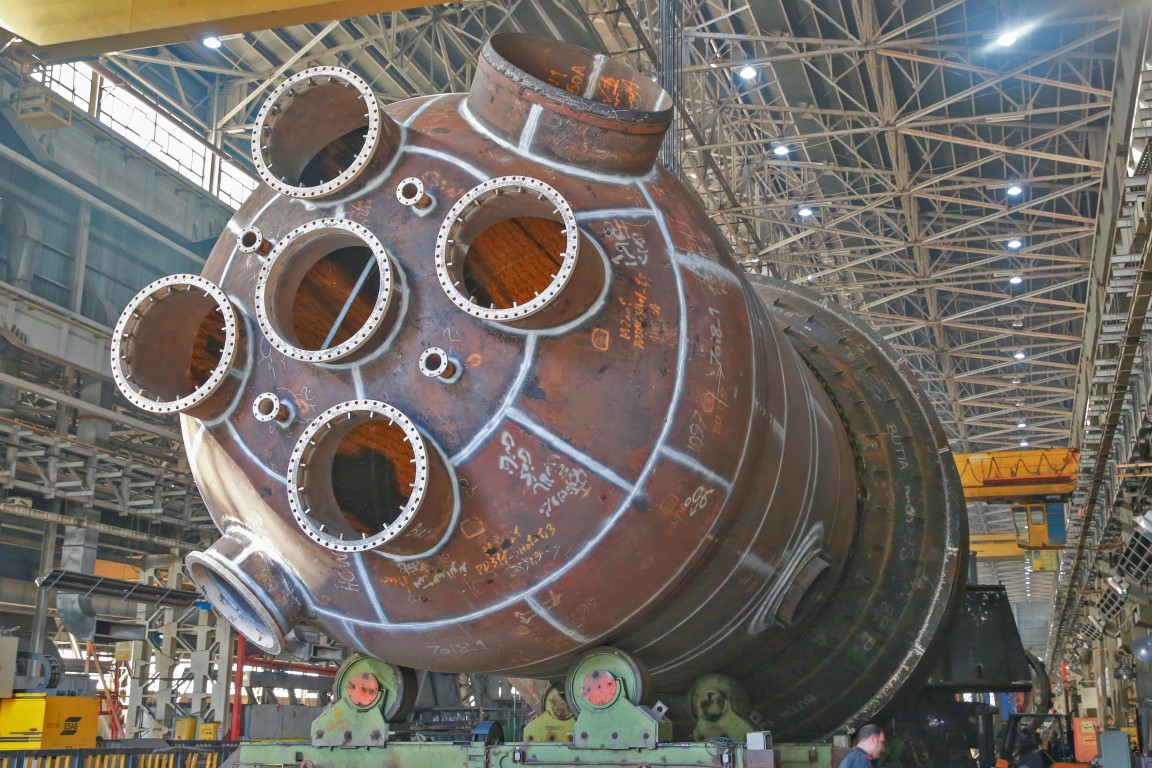
The above two reactions are very important. In fact, the same gases used in the primary iron ore regeneration process will carburize the produced iron, and as a result, if we seek to produce pure iron from the HYL process, we will encounter problems in this area. On paper, the required percentage of each hydrogen and carbon monoxide reduction gas will be 72% and 16%, respectively. Meanwhile, a part of the recovery gas used during the process will convert the sponge iron produced in the furnace into cementite without playing a role in the recovery of the primary ore. In other words, for a theoretically required amount of regeneration gas per kilogram of primary ore, a larger amount of regeneration gas is needed. The working temperature in the HYL process is about 930 degrees Celsius, which is necessary to maintain this temperature in order to achieve the required level of iron ore metallization. We said that the last two reactions have an important contribution at the end of the process. The important issue that stands out in the meantime is the endothermicity of the iron carbide production reactions, which will reduce the temperature of 930 degrees Celsius, and this is not desirable because, as mentioned, it affects the chemical properties of the final metal. It will have devastating effects. For this reason, it is necessary to optimize the conditions (including the amount of initial methane injection, maintaining the temperature of the furnace environment, the desired amount of carbide production, etc.). At the beginning, we said that HYL technology underwent two fundamental changes over time. These two changes (HYL II & HYL III) had their own characteristics; First, the generation after The HYL technology was the HYL II technology, which never went beyond an experimental design. One of the main and important reasons for this was the simultaneous evolution of the HYL III technique, which, in addition to having second-generation innovations (such as changes in the regeneration gas unit system, optimization of energy consumption, reduction of the number of furnaces from four in HUL to two and ...), the most important change in the HYL process was also included. We said that the HYL process had a static (fixed) substrate. In practice, this technology had four furnaces, where the regeneration process was divided into two parts and the carburization process was done in a separate part. HYL III technology replaced four furnaces with two furnaces (which are also known as shaft furnaces) and by modifying the consumption pattern Energy in this process is now able to produce sponge iron with a purity between 83 and 90% of iron. Sponge iron in Iran Sponge iron has a porous and spherical structure. This direct recovery product is used for about 5% of the world's steel. The use of sponge iron for iron extraction in induction furnaces started in 2009 in Iran. A factory in Yazd was one of the pioneers of this path. It should be noted that at that time they were not yet faced with the problem of scrap shortage, and perhaps for this reason they did not turn to using sponge iron before this time. The use of sponge iron first faced challenges for steel producing factories. Due to not being familiar with how to charge the furnace with the direct regeneration product and the presence of phosphorus and sulfur in it, the melting time and the amount of slag increased a lot. These issues caused factories to not like using sponge iron. After this period, with the progress made and more familiarity with this method and, most importantly, the lack of scrap, the use of sponge iron increased day by day. Construction of sponge iron recovery reactor with HYL3 technology for Iranian Butiai steel factory by Arak Machinery The sponge iron regeneration reactor with HYL3 technology and the capacity to produce 2 million tons of sponge iron per year was successfully designed and built for the first time in the country and the fourth time in the world at Arak Machinery Manufacturing for Butiai Iranian Steel Plant located in Chatroud, Kerman. Also, all the raw materials needed to make it are provided from within the country. This project actually has two parts, the operation of transporting and sending the first part of this reactor, called Cooler, was done in July, and the main part has left Arak for Kerman. The operational weight of the reactor is 2877 tons, which is installed vertically on a metal structure at a height of 45 meters in the regeneration tower, and it converts iron pellets into sponge iron by using regeneration gases (H2, CO) at a temperature of 950 degrees Celsius. It should be noted that the transportation of this reactor with cargo dimensions of 12 meters wide, 12 meters high, 40 meters long and 420 tons in weight from the origin of Arak Machinery Company to the destination of Chatroud, Kerman, has been dubbed as the highest cargo in the history of super-heavy transportation in the country. Is. Due to the long distance, the long duration of transportation (about 8 months if the conditions are favorable) and due to the importance of protecting the paint applied to the body of the equipment against atmospheric factors along the way, a polymer coating has been applied on the entire shipment. that this cover was only to protect its color and was not a reason for concealment. At the end, you can see pictures of this huge project.